Kraft pulping process is the most used pulping process in the world. In this process lignin goes into the reactions with the cooking liquor chemicals and split into fragment. These fragments dissolved with the solution and wood disintegrates into fiber. During the kraft pulping process about 80 percent of lignin, 50 percent of hemicelluloses and 10 percent of celluloses are dissolved.
In Kraft pulping process, cooking liquor chemicals are NaOH and Na2S. Although the high percentages of sodium sulfide darken the pulp color but it has huge benefits; it increase penetration of the wood, uniform cooking, low cooking time, high yield and strength. The Na2S hydrolyzed in presence of water and gives hydroxide and hydrosulfide. The reaction is reversible and can be described as below:
In kraft pulping process NaOH is the key chemicals for completed the cooking process. Temperature also plays a significant rule. The main chemical reactions in the kraft cooking process can be expressed as:
NaOH + NaSH + Wood → Na-compound + S-compound
Here the wood represents various organic compounds as like: Cellulose, Hemi-cellulose, Lignin, fats, and Resins.
The average wood chemical compositions are:
Cellulose: 40-45 %
Lignin 18-32 %
Rest are Hemicelluloses, fat, Resins
After the karft cooking process the chemical composition of the pulp are:
Cellulose: 70-75 %
Lignin 2-5 %
Rest are Hemicelluloses and others organic compounds.
At first step of the karft pulping process the white liquor penetration and diffusion into the interior of the wood chip.
When the wood chips are heated the delignification reactions starts, but the reaction rate is very slow. The specific kraft cooking temperature is generally 135-175°C in which the lignin structure and others organic compounds are broken down into small fragments and solubilized into the alkaline solution.
During the cooking process, mainly the alkali is consumed by five different ways: (1) reaction with lignin, (2) Neutralization of different organic acids (original wood acids or produced by hydrolysis) (3) reaction with resins in the wood (4) dissolution of carbohydrates and (5) adsorption by the fibers. About 60-70% of alkali is consumed by neutralizing of the organic acids; whereas about 20-30% of alkali is consumed by the degradation products of lignin.
Reactions with lignin
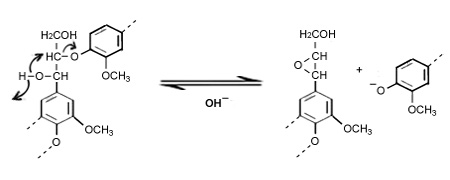
Lignin is chemically split into fragment by hydroxyl (OH and hydrosulfide (SH) ions. If the percentages of Na2S are low then the fragmentation process can be proceed through condensation reactions, either with themselves or undissolved lignin or carbohydrates; which is very difficult to remove.
By blocking the reactive groups as like hydroxyl in benzyl alcohols or alkyl ether groups hydrosulfide ion reduce the condensation reactions (sulfur combine with reacting groups and produce thiolignin). This compound easily goes into alkaline solution and removed.
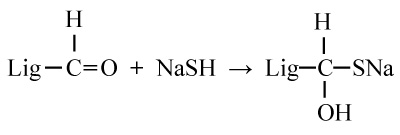
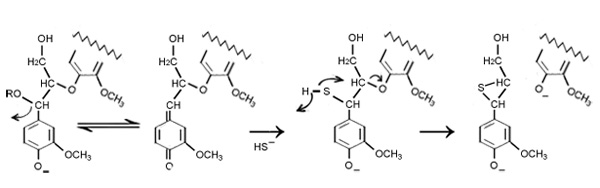
The sulphide promotes and accelerates the cleavage of the ether links in phenolic units. In alkaline conditions the carbon-carbon bond is more stable than the oxygen-carbon bonds, hence the cleavage of oxygen-carbon bonds are the most significant reaction in the cooking process. Through this reaction phenolic hydroxyl groups and carboxylic compound are produced from the cleavage of the aryl-alkyl-ether and dissolved as phenolate and carboxylate ions.
In presence of alkali, the carbohydrates (cellulose and hemicellulose) are degraded and huge amount of cooking liqor is consumed. Hemicelluloses are a polysacchrrinic compounds including galactoglucomannan, arabinoglucoronxylan, arabinos, arabinogalactane, xylan, glucoronxylan, glucomannan, glucorans, galactorons etc. During cooking process the polysacchrrinic compounds are hydrolysised and degraded into saccharine acids. For neutralization of these organic acids another portion of alkaline is consumed. In this way hemicelluloses dissolved into the pulping liquor. During kraft pulping process about 50% of hemicelluloses and 10% of celluloses are dissolved.
In the cooking reaction Most of the extractives, fat, resins are converted into soap and dissolved in the cooking liquor. This are generally floated of from the black liquor in the evaporation area and can be processed to make tall oil.
In this process many unwanted gas are produced that are non dissolvable in the black liquor such as CH3SH, CH3SCH3, CH3S2CH3 etc. sometimes these unwanted gas should be released. Again, others countable by-products are turpentine and methanol.

Very halpfull artical.
Please give me information about reaction of cl². NaoH. H2O2.Oxygen. Clo2. Waste acid. And so2 in kraft pulping
What type of Na-compound and S-compound can be expected by which chemical reaction during kraft pulping process?
please send the chemical reactions are occuring while the pulp is bleached by hot water, post oxygen, clo2, h2o2 + Naoh, clo2 + h2so4.