Oxygen Delignification is one of the most significant and well proven pulp bleaching process for ECF (elemental chlorine free) and TCF (total chlorine free) bleached pulp production. It is the first stage of the bleaching process; in this case oxygen and alkali are used to eliminate a portion of the residual lignin in the pulp after cooking. This stage is frequently recognized as a “bridging step” between cooking and final bleaching. This process is work at high temperature and pressure. The process may be operating at medium consistency or high consistency; but medium consistency system is mostly used due to easy maintaining. It can be single or multiple stages. This bleaching process is environmental friendly and cost effective.
With a single Oxygen Delignification stage, it can be reduced about 30-50% lignin and colored substances from unbleached pulp. Whereas with the external stage, it can be reduced about 60 -70%. After oxygen bleaching the lignin content may reduce to about 1.5-2% from 3-5% or kappa number 8-10.
Condition for oxygen delignification
Pulp consistency 10 – 15%
Retention time: 50 – 60 min
Temperature: 80 – 120 °C
Pressure: 600 – 800 kPa (or 6 – 8 bar)
Alkali: 20 – 25 kg/ton
pH value: above 10
Oxygen consumption: 20 – 25 kg/ton
MgSO4: 1 – 3 kg/ton
Temperatures and pressures
Oxygen is a low reactive oxidant. Therefore, oxygen delignification requires elevated temperatures from 80°C to 120°C and pressures from 6 to 8 bars for increase reactions rate. However, an unacceptable degradation of carbohydrates is shown in case of high temperature above 120°C.
NaOH and pH
Amount of NaOH and pH value is very significant for oxygen delignification. The pH value should be above 10, it is better keeping around 12 for effectiveness. If the oxygen gas bubbles are not well distributed, it may hamper on effectiveness. On the other hand, high alkali charges arises loss of yield and pulp strength.
Function of MgSO4
The transition metals, such as Mn, Cu and Fe may contain in pulp and can work as catalysts for peroxide decomposition. To reduce this negative effect MgSO4 is used. Moreover, it is used as a viscosity protector because of oxygen delignification can reduce the pulp viscosity.
Advantages of oxygen delignification
Environmental friendly
Oxygen Delignification process is environmental friendly process which is helping to reduce the uses of chlorinated chemicals in next steps. These chlorinated chemicals are produced some organic halide compound which is very poisonous for our health. The other environmental benefits are lower BOD, COD and fewer colors in effluent, as all the effluent from the oxygen bleaching system is recycled back to the recovery boiler.
Cost effective
Oxygen Delignification significantly reduced the consumption of bleaching chemicals (such as chlorine gas, chlorine dioxide, ozone, hydrogen peroxide) of next stages. Although the installation cost of oxygen bleaching plant is expensive, but the overall oxygen production cost is extremely minimal.
All the effluent containing spent chemicals and reaction products from the oxygen bleaching process is recycled back to the recovery boiler. Whereas without this stage all the effluent would go to wastewater treatment plant due to presence of corrosive chlorinated compounds, which might be harmful for recovery boiler. Hence, it reduced chemical consumption cost.
By optimizing the cooking kappa number in cooperation with the oxygen delignification, it can enhance pulp yield percentage and maintained pulp strength. After this stage there are lower shives and extractives content.
Disadvantages of Oxygen Delignification
Oxygen bleaching process is less selective compared to others bleaching agent, as the process undergoes with the free radical reactions system. This can lead to significant degradation of polysaccharides (by hydroxyl radicals); consequent of carbohydrate reactions the viscosity of pulp and fiber strength could reduce. It is a drawback of oxygen bleaching.
Another significant disadvantage of Oxygen delignification is that it is weak reactive oxidant. Therefore, it required alkaline conditions, high temperature and pressure to obtain a reasonable reaction rate. Raising the alkali charge also creates a negative effect.
Moreover, primary high installation cost of oxygen delignification is another drawback.
Lignin Reactions during oxygen bleaching
At lowest energy state Oxygen molecule contains two unpaired electrons; each of these electrons has an affinity to opposite spin of other electrons. Therefore it can act as a free radical. Although at normal temperature it will un-reactive, but at higher temperatures and alkaline condition it has a strong tendency to react with organic compounds.
At initiated state phenolic hydroxyl group in lignin reacts with alkali to generate a phenolate ion. The phenolate ion then reacts with oxygen to form a resonance-stabilized phenoxy radical and a superoxide anion.
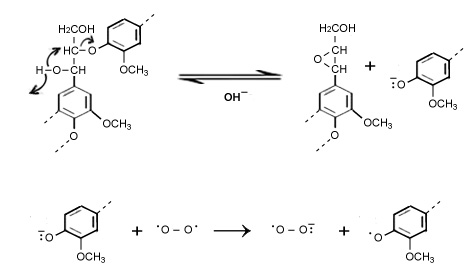
Then the phenoxy radical undergo reaction with themselves or with oxygen species radicals for example hydroxyl (HO•), hydroperoxy (HOO•) and superoxide (O2•–) and produce different organic acids, carbon dioxide and other lower molecular mass organic compounds through side chain elimination, ring opening and demethoxylation reactions.

One of the biggest draw back of ODL is that it increases the solid load to recovery cycle. As most of the recovery boilers are running at full capacity so introduction of ODL system will lower down overall production. But in case you have margin in evaporator plant , Recovery boiler and re causticising plant then ODL is most promising stage. But as a caution the post washing after ODL must be very efficient otherwise carryover of oxidised lignin will consume bleaching chemicals and all benefits will be lost.
It is more beneficial for soft wood because its kappa is mainly due to lignin. But in hard wood kappa is mainly due to Hexauronic acid which cannot be removed in ODL process. So 50 – 60 % kappa reduction can be achieved in soft wood but it limits to 30 – 35% reduction in tropical hardwood and eucalyptus.
I have vast experience in running ODL plant for various raw materials. Any one having any specific doubt can contact me in e mail ID atsvillage1974@gmail.com
Thanks for your important information. In our mills, there is no option to recover it; always goes to drain. If you want to share information you can write in this blog.
Dear Sir
Re : ODL for bagasse pulp
The following are the main issues in ODL for bagasse pulp. So one must take care for the same :
1. Being short fibre , chances of zero fibre entering the liquor cycle is high which ultimately chokes the spray nozzles of washers / displacement press. So need to have on line screens.
2. bagasse pulp is slow in drainage so difficult to get higher consistency after washers / press. This results in liquor carry over to bleaching stage.
At present our soda loss at final washing stage is approx. 25kg/ton of pulp. So in this case how ODL plant will work at present we do not have ODL plant.
Everything you said about oxygen deliginfication stage is true. ODL have strong and weak points as you were referring to. Therefore we (two romanian pulp specialist engineers together with an Professor specialist in catalysts) tried another extraordinary bleaching method: polyoxomethalates delignification and in two POM stages we obtained an 82 degree of brightness. While all bleaching steps need rough bleaching condition, (remember ODL at high pressure, high temperature, alkali consumption and a need of very good washing after ODL, POM transform ligning (to which is very selective) almost into CO2 and H2O, the POM stage need 60 degree Celsius at atmospherically pressure. More than convenient. The POM steps effluents are almost uncolored. More than this, the POM can and should be reused so the liquid containg POM after POM stage will be reused in the POM stage, this way the need of fresh POM is 5 time reduced. So I asked you ALL. What is yours comments about this?
I mention that we managed even an industrial trial at Somes company (on the period when this company works of course, because now is in bankruptcy). If anyone is interested in this application which reduce two-three times the bleaching costs, do not reduce the bleached pulp physical – mechanical characteristics (no mater soft or hard wsood pulp) and gives to the bleached pulp producers the opportunity to obtain bleached pulp with an extraordinary friendly environmentally method, can contact us at dutucgheorghe@yahoo.com.
I would love to bear more about it…. thanks
Dear sir
Please explain me what is the chemical reaction during oxygen delignification.
Explain with chemical rection of ODL
I am interested in the advantages/disadvantages of a single stage reactor versus a 2 stage reactor. Can you give me more information on this?
We work in single reaction. Two reaction is the same as single but you need single reactor litle more bigger. Two reactor give you more time reaction. But cost of two reactor and work with two reacror more expensive. Result the same.
I also interested in reaction during delignification.
can anybody discuss and write about the reaction and Importance of Na2S present in white liquor.
How match chemical reaction old plant hard wood Pulp mill
Is the oxygen bleaching process patented and if so, who are the owners.
Oxygen bleaching process patent in Russia 1964. So it is free for use.
Please to explain How to separate mg metal and liquor in soda recovery plant
Pingback: What filter should I use with my Chemex? – The Brew and Buzz
We are paper pulp manufacturer and our batch composition as under ;
Pulp raw material = 5000 Lit
Starch = 500 Kg
Cooking 20-30 minutes at 95 C
Sizing agent = 20-25 Kg
Product reacived = semi thick
We want to dilute it and reduce its viscosity, what Chemical should be added which should not affect our product quality and viscosity is reduced.