The Kappa number of pulp is an indication of the lignin content or bleachability of pulp. We use it to estimate the required amount of bleach chemicals to achieve targeted brightness. If the kappa number value is higher then, the required bleaching chemicals are also higher. On the other hand low kappa pulps are easier to bleach. The KMnO4 not only oxidized the lignin, but also oxidized the others compound. Therefore it will raise the consumption of KMnO4. Consequently raises the Kappa number of pulp. It is almost proportional to the residual lignin content of the pulp. The kappa number depends on digestion system, wood species and delignification procedure. Normally the kappa number of soft wood is higher than hard wood. Oxygen delignification reduces the bleaching consumption.
Principle
The kappa number is one of the significant parameters for pulp production. It is determined as the volume of N/10 KMnO4 solution consumed by one gram of air-dried pulp in an acidic medium. The KMnO4 solution is spent due to reaction with lignin and others oxidized compounds within a given time.
MnO4– + 8H+ → Mn2+ + 4H2O
MnO4– + 4H+ → MnO2 + 2H2O
The KMnO4 solution is added such an amount so that about 50% permanganate is left unconsumed before adding the thiosulfate solution and potassium iodide. The excess volume of KMnO4 solution is measured by titrating with standard thiosulfate solution after adding an excess of potassium iodide.
2 MnO4– + 10I– + 16H+ → 2Mn2+ + 5I2 + 8H2O
MnO2 + 4H+ + 2I– → Mn2+ + 2H2O +I2
2S2O32- + I2 → S4O62- + 2I–
Test procedure:
- Collect the pulp sample and air dry them.
- Take 1gm air-dried pulp sample and 650ml distilled water into a two liter beaker. Disintegrate the pulp with this water so that no fiber bundles exist.
- Add 25ml 4/N H2SO4 acid.
- Add 25 ml N/10 KMnO4 solution then wait for 5 minutes.
- Add 5ml 1N KI.
- Then, titration free iodine with N/10 Na2S2O3 solution using starch indicator.
- Carry out a blank titration following the same procedure as above omitting the sample.
Kappa number calculation
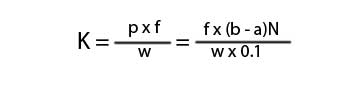
Here,
K = kappa number.
p = amount of permanganate actually consumed by the sample.
f = factor for correction to a 50% permanganate consumption.
a = amount of the thiosulfate consumed by the sample.
b = amount of the thiosulfate consumed in blank titration.
N = Normality of the thiosulfate.
The disadvantages of this method are that:-
- The iodine is volatile compound which can create an error. So a blank experiment is needed to reduce error due to high volatility of iodine.
- The reaction should be kept under weekly acidic condition.
- The reaction time may causes errors in determining kappa number for various pulps.
- Without lignin other compound like hexenuronic acid in bleached pulp also oxidized.
- Reaction temperature should be kept 25⁰C throughout the test of kappa number of pulp. Otherwise it can create errors.
I want to know the name of author,please!!!
Check your inbox
I don’t know procedure No. 6 mean
titration with free iodine.
Please explain me.
Thank you
Can I get a full report answer of this pls?
Can you please link me to a university in Canada where I can do my bench work.
Best regards.
The concentration of 25 mL H2SO4 is not precise in the test procedure point number 3 please check and make it correct
Kappa number 10-40 online sensor